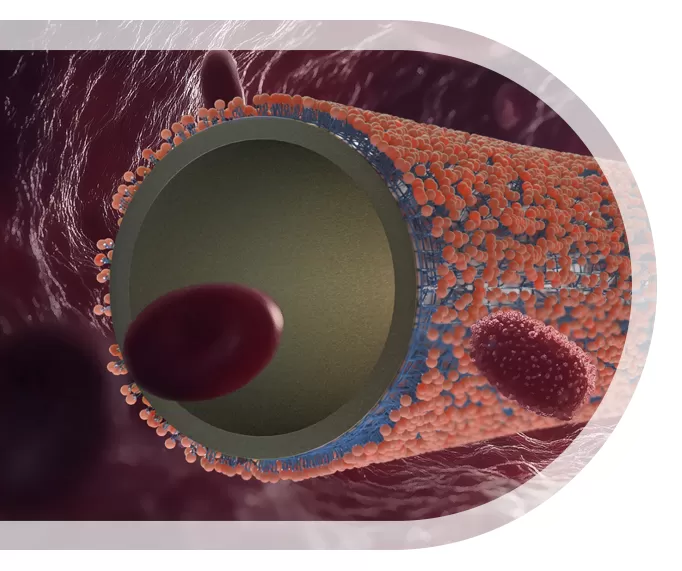
The SILVERMARS®coatings are well-established medical coatings that mimic the outer layer of cell membranes, thereby inherently possessing biocompatibility and endowing the device surface with antithrombotic, anti-inflammatory, antimicrobial, and hydrophilic properties. We invite you to discover our comprehensive range of customized coating solutions designed to optimize clinical outcomes.
SILVERMARS® phosphorylcholine coating series meets diverse clinical needs:
M Series: applicable in a wide range of scenarios;
MP Series: plus version of M series, tailored for surfaces/structures with coating challenges;
MF Series: ideal for short-term implantation scenarios;
MH Series: hydrolysis-resistant, perfect for urological application scenarios.
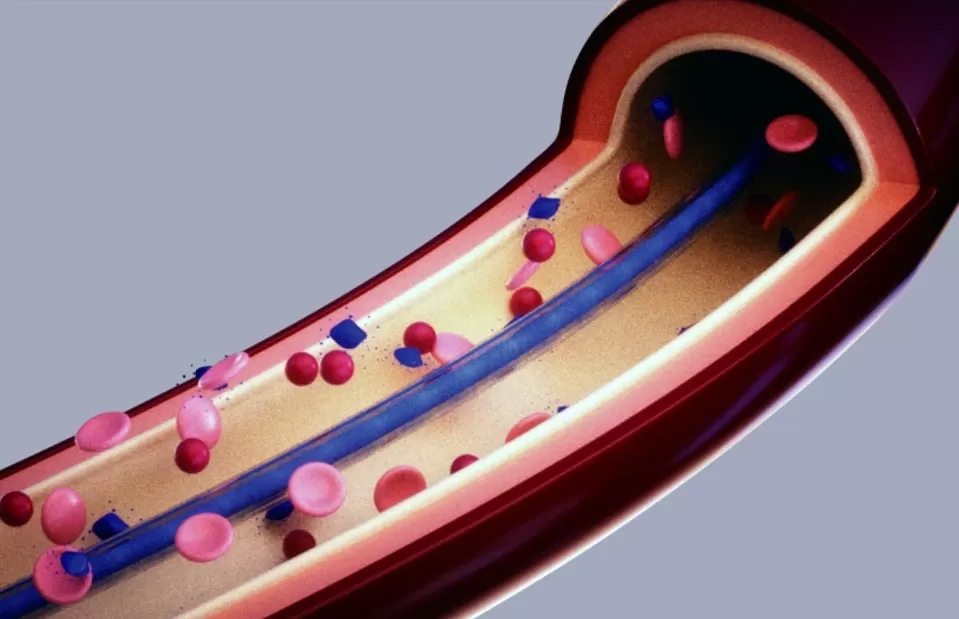
The SILVERMARS® biomedical coating series, built upon a 2‐Methacryloyloxyethyl Phosphorylcholine (MPC polymer), modifies medical device surfaces by leveraging their zwitterionic nature, which features an equal distribution of positive and negative charges within each molecule. As a biocompatible coating, it offers excellent antibiofouling capability, effectively resisting nonspecific adhesion of proteins, cells, and bacteria. By mimicking the natural structure of the cell membrane's outer leaflet, this advanced SILVERMARS® phosphorylcholine coating creates a highly lubricious and bio-inert surface that minimizes immune response and thrombosis.
Bionic Structure Enhanced Biocompatibility:
Phosphorylcholine coating enhances compatibility, minimizing inflammatory response and device rejection.
Antifouling Properties:
Phosphorylcholine coating effectively resists nonspecific adsorption of proteins, cells, and bacteria.
By creating a biomimetic surface, our phosphorylcholine coating prevents coagulation and thrombosis formation.
Heparin-Sensitive Friendly:
Safe and compatible phosphorylcholine coating for individuals sensitive to heparin.
Strong Durability:
Ensures sustained and long-term phosphorylcholine coating performance via high durability.
| Phosphorylcholine Coating |  | Heparin Coating |
| Molecular Structure | ||
| Physical Anticoagulation | Anticoagulation Principle | Drug - Based Anticoagulation |
× All individuals | Biological Rejection Applicable Population | √ 5% of Those Intolerant to Anti- rejection Medications |
Nanoscale | Synthesis Complexity Coating Thickness |
Micron - Scale |
  | Coating Difficulty |     |
| Stable (Synthetic & Mass - produced) | Cost Stability | Unstable (Susceptible to Raw Material Cost Volatility) |
× | Overall Cost Involvement of Drug - Device Combination |
√ |
 | Regulatory Approval Duration for Related Products |     |
Multi-Substrate Compatibility
Hemocompatibility
Protein Adhesion and Encrustation Resistance
Antimicrobial Activity
Nano-Scale Coating Thickness

Uncoated

Coated
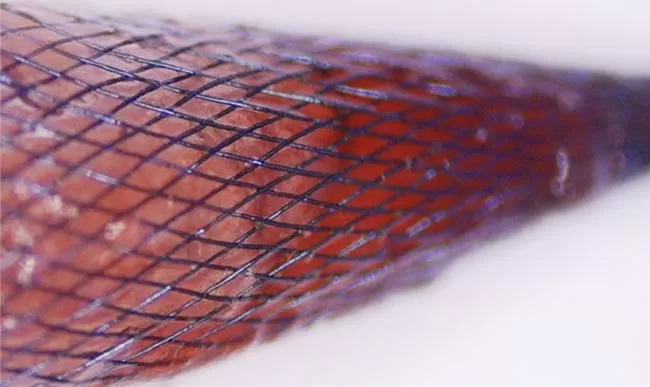
Uncoated

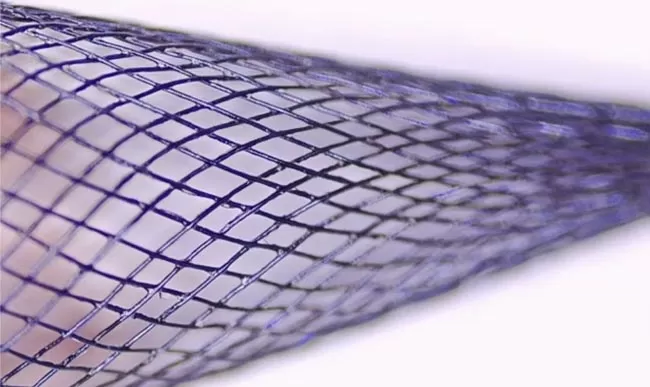
Coated
Silvermars specializes in high-performance phosphorylcholine coating solutions specifically engineered for implantable and interventional medical devices necessitating properties like biomimicry, anticoagulation, anti-encrustation, and antifouling.

Contact SILVERMARS for More Information
Email:
info@silvermarstech.comTel:
+8618321319377Address: